Cost Reduction, Precision Improvement, and Stable Delivery: What Challenges Does Multi-Type Sensor Integration Solve?
Jun 04,2025
In the era of rapid development in smart healthcare and minimally invasive interventions, multi-type sensor integration has become a key technology driving the intelligent and data-driven upgrading of medical devices. However, the challenges in this process go far beyond technical integration itself. How to embed multiple high-precision sensors in a tiny space? How to ensure stable output and reliability? How to address the rising international trade barriers? Diagsensor is delivering remarkable answers through a series of practices.
01 Market Trends and Industry Challenges
With the increasing popularity of minimally invasive surgery, the demand for interventional consumables in China is huge, with an annual growth rate expected to be 12%-15%. Medical devices are becoming increasingly dependent on sensors. The integrated application of multi-type sensors enables real-time collection of multi-dimensional data such as temperature, pressure, and positioning, assisting clinicians in making more precise decisions and improving surgical safety. However, integrating multi-type sensors into slender structures like catheters requires solving problems such as size, power consumption, signal interference, as well as engineering challenges in packaging, interconnection, and material compatibility.
In 2025, the trade relationship between the U.S. and China has been complex. The U.S. imposed a series of tariffs on Chinese goods, including a "reciprocal tariff" starting at 10% and rising to 84% at one point. Through negotiations, the situation has since adjusted. The current U.S. tariff on Chinese goods, with implications for medical device imports, results from a combination of various policies. These tariff changes have increased procurement costs and created uncertainties in supply, making domestic substitution a key focus for the medical device industry.

02 Technical Breakthroughs: Diagsensor’s Integration Capabilities
✅ Independent R&D of Core Sensors to Promote Domestic Substitution
Facing the increasingly severe international situation, Diagsensor has proactively prepared by focusing on the research and development of micro-medical sensors (e.g., pressure, temperature, electromagnetic sensors) and their cutting-edge integration. The company has the technical capability to complete packaging and assembly within catheter structures with an outer diameter (OD) of 0.5mm or smaller.
These sensors offer advantages such as small size, high sensitivity, and stable signal output, with performance comparable to imported products, while effectively reducing overall system costs and breaking reliance on overseas supply chains.

Application Scenarios of Sensors:
Pressure Sensors: For real-time intraoperative monitoring of blood pressure, intraocular pressure, intracranial pressure, intrauterine pressure, gastric pressure, bladder pressure, etc.
Temperature Sensors: For medical measurements of core body temperature, cardiac chamber temperature, blood temperature, tympanic membrane temperature, etc.
Magnetic Positioning Sensors: For 3D electromagnetic positioning and magnetoelectric composite positioning, suitable for guiding complex pathways.
✅ High-Precision Manufacturing and System Processes to Ensure Integration Stability and Performance Reliability
Diagsensor not only provides single-sensor packaging services but also excels in integrating temperature, pressure, and electromagnetic positioning sensors into one system. Achieving high-density integration of multi-type devices within a millimeter-scale space not only tests the miniaturization design capability of the sensors themselves but also places extremely high demands on manufacturing processes, assembly precision, and system-level coordination. Diagsensor Medical possesses a complete minimally invasive device processing platform to ensure that sensor integration systems exhibit excellent functional stability and reliability.
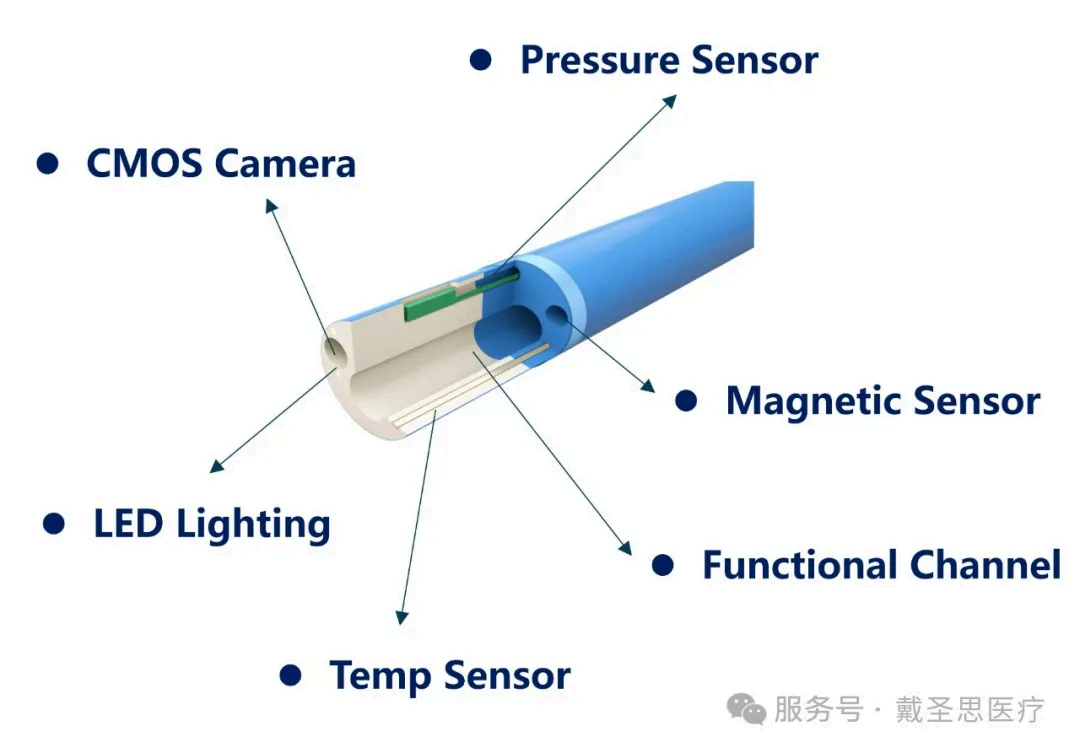
Core Capabilities:
🔹 Ultra-micro sensor component design and integration – Solutions for integrating ultra-small devices (e.g., temperature sensors and magnetic positioning sensors with a minimum OD of 0.2mm).
🔹 Precision welding technology platform – Covers processes such as minimum wire diameter (0.009mm) welding, dissimilar metal welding, FPC welding, and electrode ring-to-wire welding.
🔹 High-precision laser processing system – Laser welding and cutting technologies ensure assembly structure stability and dimensional consistency.
🔹 Micro-electrode and multi-sensor assembly processes – Supports complex structural treatments such as multi-strand wire threading, multi-lumen tube assembly, variable-diameter catheter 套接 (socket joint), and memory alloy shaping.
🔹 High-reliability bonding and packaging technologies – Adaptable to multi-material packaging (epoxy, UV, silicone, etc.) for complex bonding scenarios such as multi-balloon and high-density device applications.
🔹 Precision catheter forming and processing capabilities – Including tip forming, stepped grinding, punching, flanging, crimping, and other key structural treatments for minimally invasive catheters.
🔹 System-level testing and calibration capabilities – Covers airtightness testing after pressure catheter integration, temperature/pressure signal calibration, and finished product performance verification.

Diagsensor Medical’s systematic and high-precision manufacturing platform helps intelligent medical devices transition from "concept" to "mass production."
03 Application Scenarios
Diagsensor’s sensor integration technology has been widely applied in:
Ablation catheters
Mapping catheters
Urological catheters
Ultrasound intervention devices
Miniature endoscope systems
By real-time acquiring temperature, pressure, and positioning data at the catheter tip, surgical precision is significantly improved, treatment efficiency is enhanced, and complication risks are reduced.

04 Company Introduction
Diagsensor specializes in sensor design, system integration, and minimally invasive device manufacturing for the precision medicine field, with leading advantages in the independent R&D and integration processes of ultra-miniature magnetic positioning, temperature, and pressure core devices.
The company not only provides customized sensor solutions but also offers one-stop manufacturing services covering the entire process from minimally invasive device design and prototyping to mass production, assisting clients in rapidly launching high-end medical products.
Headquartered in Shenzhen with a R&D center, Diagsensor also operates a 5000㎡ GMP/FDA-standard manufacturing base in Taizhou, Zhejiang, certified under the ISO 13485 quality management system. The company continues to provide professional and reliable products and solutions to global clients.
🌍 Drive micro-manufacturing to excellence.
Global Supplier of Core Components and Ultra-Precision Manufacturing for Minimally Invasive Interventional Devices

Jun 04,2025
Category:
Press Releases
Related Information
Nov 04,2025
Sep 28,2025



